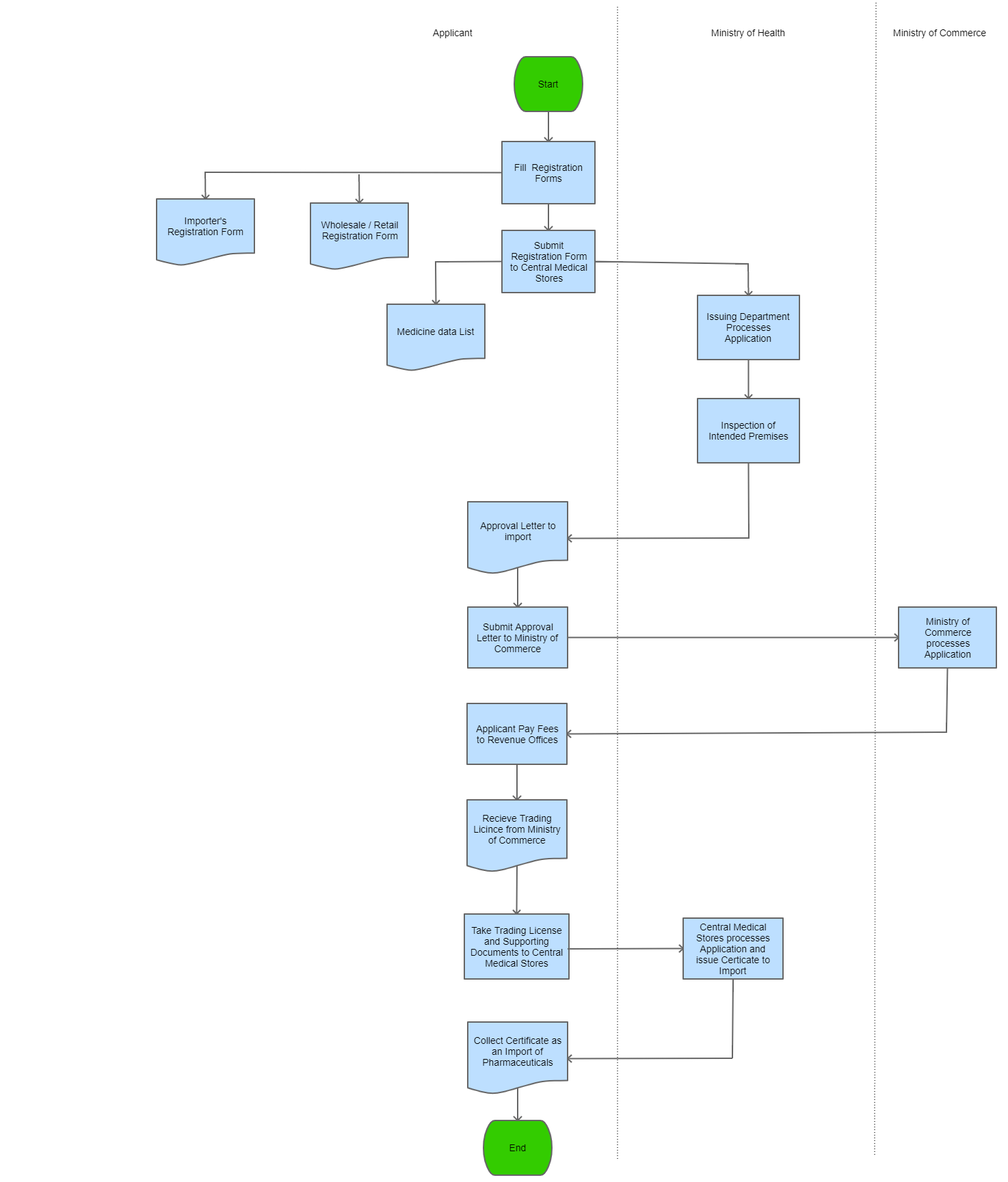
View Procedure
| Procedure Name | Registration of Pharmaceuticals Importers | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
Required Documents
Process Steps
| |||||||||||||||||||||||||||||
| Category | Import/Export |
The following form/s are used in this procedure
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| Swaziland Medicine Importer Registration Form | Application to register as an importer of pharmaceuticals | 16-03-2020 | 16-03-2020 | ||
| Swaziland Medicine Retail Registration Form | Application Form to register as an importer of Pharmaceuticals | 01-06-2020 | 01-06-2020 | ||
| Registration Form Pharmaceutical Wholesale Application | Application Form to register as an importer of Pharmaceuticals | 01-06-2020 | 01-06-2020 |
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| No results found. | |||||||